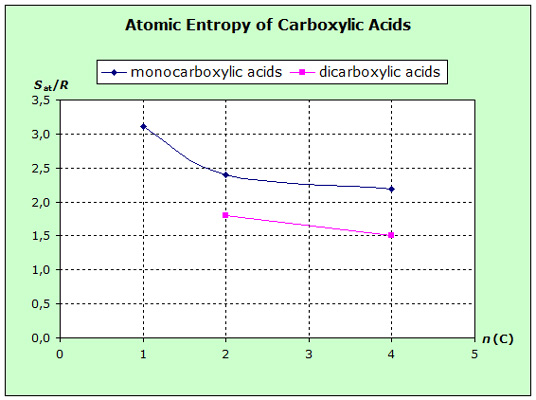
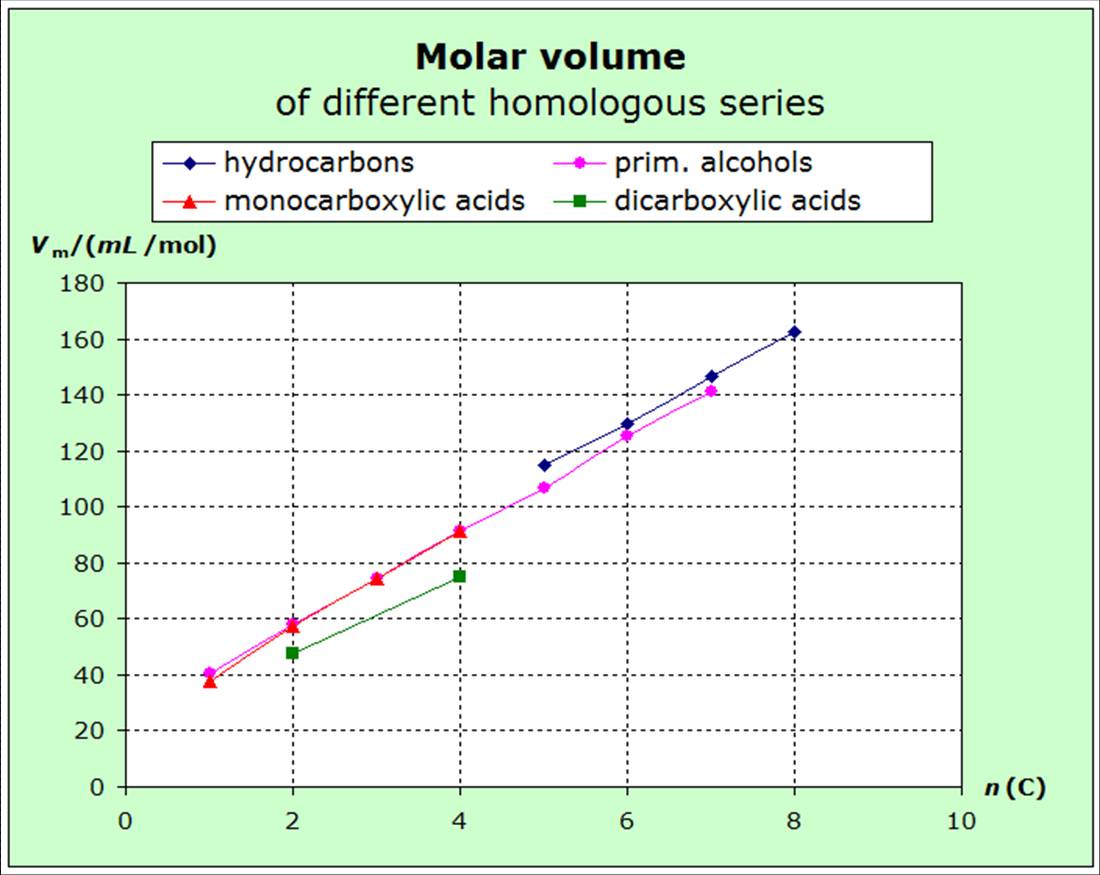
The atomic entropies of organic compounds and those of
salt-like materials show a clearly different behavior. This is due to the different forces of inner cohesion.
In the case of the salts electrovalences occur
between the ions and strong Coulomb-forces are present, while atomic bondings within
the organic substances lead to van-der-Waals-forces
between the stationary and/or temporary dipoles.
Since Coulomb-forces
become larger with smaller ion distances, the average particle distances of the salts had been calculated and shown in an own chart. (see 3.3 Inorganic compounds)
Van-der-Waals-forces however rise with
increasing volume (exact surface) of the molecules. The following diagrams show that the
entropies decrease within the homologous series with increasing van-der-Waals-forces, i.e. with increasing molecular volume.
The diagrams show the atomic entropies for liquid hydrocarbons, primary alcohols and
for some mono- and dicarbonic acids and additionally separate
diagrams for molecular volumes. |
|
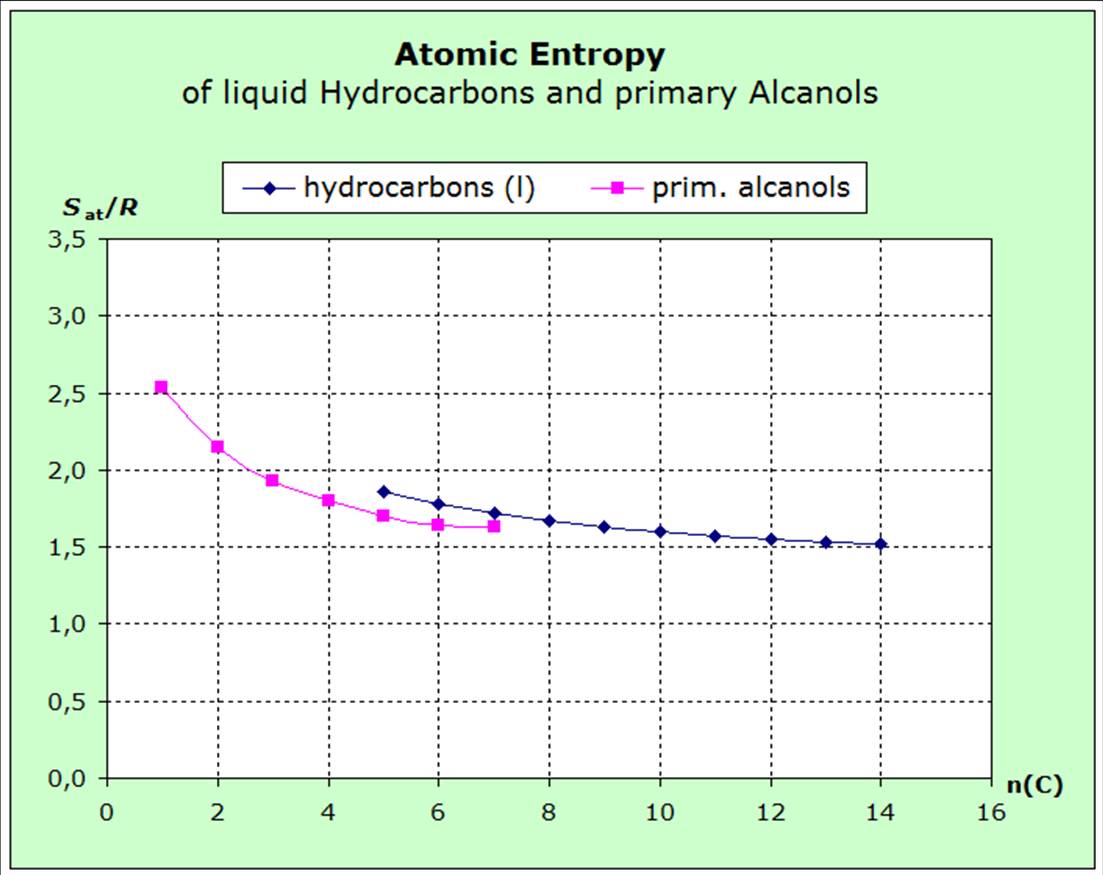
The decrease of the atomic entropies of the saturated liquid hydrocarbons from pentane to octane shows that the forces acting between the molecules increase in these substances as the number of carbon atoms increases.
The behavior of the entropy of the primary alcohols also shows this behavior.
However, the course of the average atomic mass also changes with the chain length.
One alcoholic group added to the 6 C-atoms of hexane leads to Hexanol and reduces the entropy value:
Hexane - Sat/R=
1,77
Hexanol - Sat/R= 1,64
The additional oxgene atom in hexanol increases the atomic average mass for about 13% which would cause a higher entropy value. However, the polar OH-group is linked with enlarged van-der-Waals-forces which would lower the entropy. The measured values show that the impact of the forces is obviously of greater importance rather than the impact of the average mass. The force rule takes precedence over the mass rule and has therefore been stated before the mass rule. |